Reviewed by Anurag Mishra (Sr. Technical Consultant)

Stress corrosion cracking is one type of material deterioration that takes place where a susceptible material is subjected to a corrosive environment under tensile stress. Stress corrosion cracking has the potential to initiate sudden and catastrophic failures without any prior warning, and hence, is a matter of major concern in various industries, including oil and gas, chemical processing, marine, and power generation.
It usually starts from minute cracks or imperfections, which increase in size with the synergistic effect of corrosion and stress. Material composition, environmental conditions, and stress levels determine its magnitude, and thus, preventive strategies along with cautious material choice are essential in engineering design.
In this article, we will discuss everything about stress corrosion cracking in stainless steel, brass, and other materials. Additionally, we will walk through the SCC mechanism, how it works, how it is tested, and the primary reason behind this cracking.
Stress corrosion cracking is defined as a failure of a metal due to the combined impacts of static tensile stress and chemical environment initiates and propagates fine cracks. It is distinguished by high aspect ratio cracks that cause component failures and correspond with certain environmental conditions, including hydrogen sulphide and chloride ions.
Stress corrosion cracking is the development of crack growth in a susceptible material under the combined action of tensile stress and a particular corrosive environment. It is an insidious process since it can take place below the yield strength of the material and frequently without considerable overall corrosion.
Localized corrosion (e.g., pitting, intergranular attack) forms micro-defects or soft spots.
Applied or residual tensile stresses are localized at the defects.
Corroding species (e.g., chlorides, hydroxides) enter the crack tip.
Stressed metal is anodically dissolved or hydrogen-embrittled at the crack tip.
Incremental advance of the crack as corrosion dissolves metal in front of the tip and stress increases the crack opening.
The crack is of a size at which the available cross-section is no longer able to support the load.
Sudden brittle fracture results, usually with minimal warning beforehand.
Material: Some alloys (e.g., austenitic stainless steels in chlorides, high-strength steels in alkaline conditions) are more susceptible.
Environment: The presence of specific ions, temperature, and pH has a major influence on susceptibility.
Stress: Both applied external loads and welding, forming, or machining residual stresses play a role.
Although SCC involves simultaneous stress and corrosion, evaluating a material’s surface protection is important in preventing initiation. For instance, accelerated corrosion testing using a Cyclic Salt Spray Chamber (ASTM B85) provides insight into how quickly coatings or bare metals degrade in chloride-laden environments, which are key in SCC initiation.
A Stress Corrosion Cracking (SCC) diagram demonstrates the material susceptibility, tensile stress, corrosive environment, and crack propagation. It determines how a material fractures because of the combined action of tensile stress and the corrosive environment.
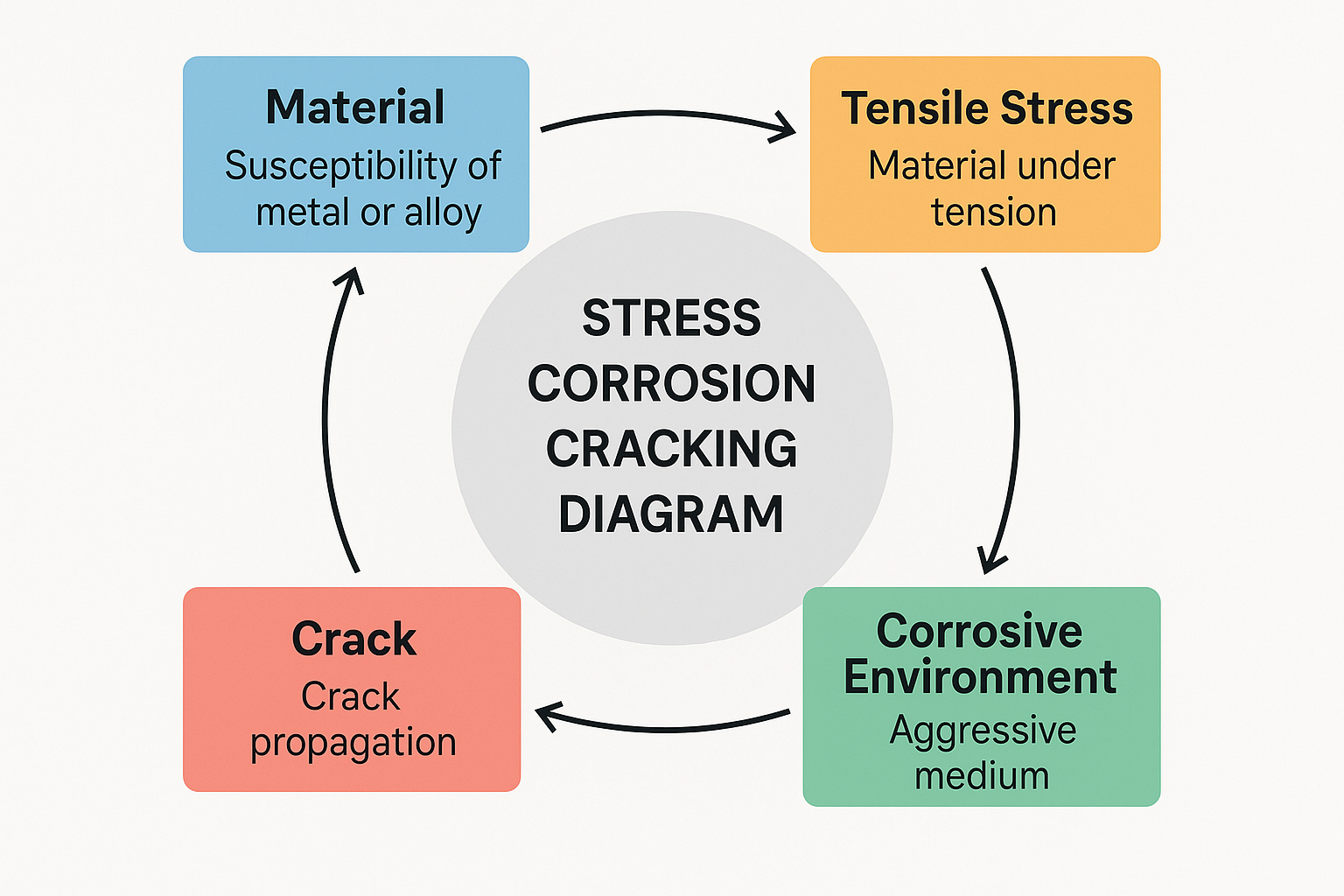
Material: This refers to the metal or alloy prone to SCC. The properties of the material, including its microstructure and corrosion resistance, determine its susceptibility to SCC.
Tensile Stress: SCC depends on tensile stress, i.e., the material is under tension, stretching it. This stress may be residual (pre-existing from manufacture) or applied (due to outside forces).
Corrosive Environment: A corrosive material is required for SCC to take place. The corrosive material acts on the stressed material, corroding it and inducing crack development. Various materials are prone to various corrosive materials. Chlorides, for instance, can lead to SCC in austenitic stainless steels.
Crack: The crack is the visible expression of SCC. It may be exposed on the surface or travel internally, finally causing failure.
Stress corrosion cracking of stainless steel is a type of environmentally assisted cracking that results when tensile strength and corrosive environment interact to make cracks extend in the material.
A common example of stress corrosion cracking in stainless steel is chloride-induced SCC, which mainly affects austenitic stainless steels. This type of cracking occurs when the metal is subjected to tensile stress in the presence of chlorides, such as salt ions, often found in seawater, hot water systems, or industrial environments.
For instance, in hot water systems and heat exchangers, austenitic stainless steel can develop fine cracks that grow over time due to the combined effects of mechanical stress and exposure to chloride ions. These cracks often initiate at microscopic defects or surface flaws and can propagate rapidly, leading to unexpected failure of components even when corrosion appears minimal on the surface.
Stress corrosion cracking of brass is a condition where brass parts crack under the combined action of tensile stress and corrosive environment, which frequently involves ammonia.
Example: Seasonal cracking – It was first noted in brass cartridge cases used by the British Army in India in the 19th century. With the onset of the Monsoon season, the cases that were left in stables cracked, especially at regions of high stress, such as the crimped portion close to the bullet. This was due to a mix of manufacturing residual stresses.
Stress Corrosion Cracking test consists of several testing methods concerning different standards such as ASTM G139, ASTM G103, ASTM G35, ISO 6957, and NACE TM0198.
ASTM G139 - This test procedure included methods for testing stress corrosion cracking (SCC) resistance by breaking load test method, a principle that employs residual strength as a measure of damage development (in this instance, environmentally assisted cracking).
ASTM G103 - ASTM G103 is a guide for testing the resistance of low copper 7XXX series aluminum-zinc-magnesium-copper alloys to stress corrosion cracking, in particular within a boiling 6% sodium chloride solution. This test is mainly applied to smooth, non-welded or welded specimens under static tensile stress.
ASTM G35 - ASTM G35 is a standard practice for testing the intergranular stress corrosion cracking (SCC) susceptibility of stainless steels with a polythionic acid solution at ambient temperature. Similar durability checks for plastics can be done using an Environmental Stress Cracking Resistance (E.S.C.R.) Tester.
ISO 6957 - ISO 6957 is an international standard that defines a stress corrosion cracking test for copper alloys, employing an ammoniacal atmosphere. The test is to determine whether applied or residual stresses in copper alloy products may lead to them failing in service by means of SCC. The test severity can be varied by modifying the pH of the solution which creates the ammoniacal atmosphere.
NACE TM0198 - NACE TM0198 is a recommended test method based on slow strain rate testing (SSRT) to screen corrosion-resistant alloys (CRAs) for susceptibility to stress corrosion cracking (SCC) in high-temperature sour oilfield environments. It is particularly applicable to stainless steels and nickel-based alloys.
Stress corrosion cracking occurs when a susceptible material is exposed to a specific corrosive environment while under tensile stress. The combined effect causes microscopic cracks to form and grow, often leading to sudden failure, even when overall corrosion appears minimal.
A material susceptible to SCC in an environment: Various materials show susceptibility to SCC in various corrosive environments. For example:
Austenitic stainless steels are also especially susceptible to chlorides, particularly at high temperatures.
Copper alloys suffer from cracking in ammonia or ammoniacal solutions, which is a well-known old phenomenon of "season cracking".
Carbon steels can suffer from SCC in hydroxide, nitrate, carbonate, bicarbonate, and acidic hydrogen sulfide-containing environments.
A specific corrosive environment: The environment should have chemical species that are capable of interacting with the susceptible material in a manner favorable to crack initiation and propagation. Chloride-containing solutions, ammonia, caustic solutions, hydrogen sulfide, and acids are some examples of corrosive environments.
Tensile stress: Tensile stress might be applied externally during service or be residual stress trapped within the material due to manufacturing processes such as welding, cold working, or heat treatment. SCC generally occurs at stress levels below the yield strength of the material and is thus very sinister as it causes sudden, brittle-like failures in otherwise ductile materials.
Stress corrosion cracking refers to the simultaneous interaction of tensile stress and corrosive environments, which can lead to the rapid failure of materials. Understanding its definition, mechanisms, and contributing factors—supported by examples and diagrams—allows for accurate detection. Following appropriate testing protocols and implementing countermeasures are essential to reduce risks and ensure material reliability in engineering designs.